
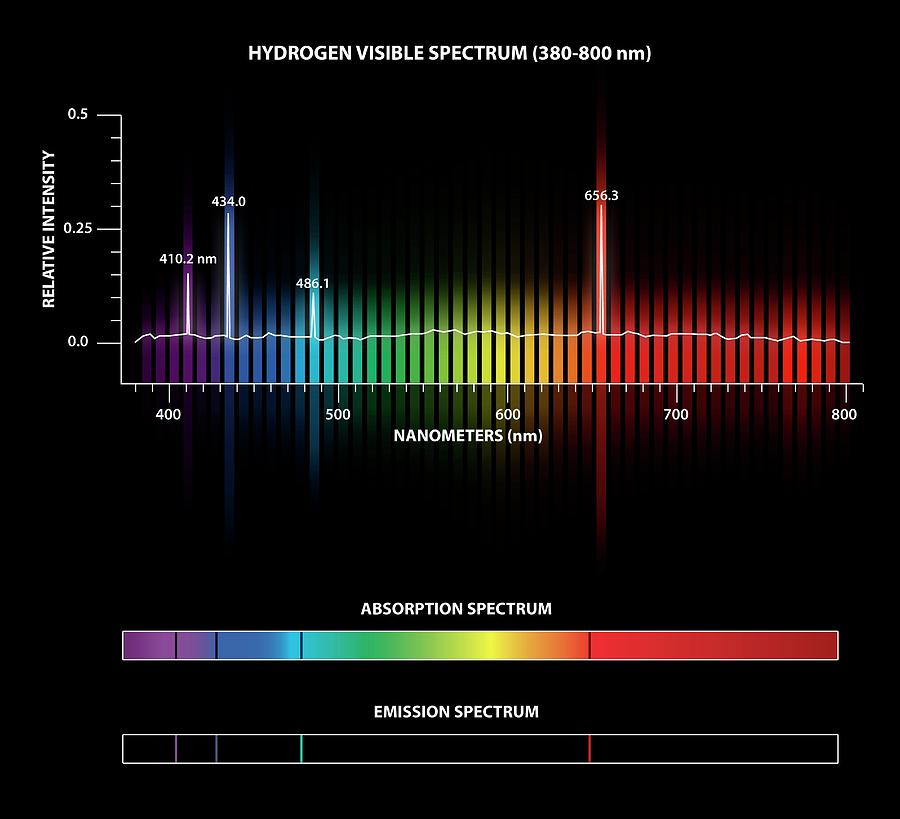
Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed by atomic absorption. If boron emits radiation at 518 nm, what color will boron impart to a flame. Which can be related to Einstein’s equationīoth atomic emission and atomic absorption spectroscopy can be used to analyze samples. Plank proposed radiation emitted energy in discrete packets (quanta), ABSORPTION SPECTRA Optical absorption and photoeffect in B - rhombohedric boron. When we say unique, we mean that all hydrogen atoms have the same spectrum, and this spectrum is different from all helium atoms and all boron atoms et cetera. Model 2 -Emission Spectra for Hydrogen and Boron Atoms Hydrogen Slits Prism Blue- Blue- Violet. Go to the rest of the lab stations and repeat for each element (helium, neon, argon. Boron : production, structure, and properties. absorption spectra of hydrogen and boron would look like. All other colors observed in the spectrum of hydrogen are a result of electron transitions from other energy levels (4, 5 and 6) to energy level 2. It relates the distribution to the thermal temperature of the system (as opposed to electronic temperature, vibrational temperature, or rotational temperature). Astronomical spectra can be combination of absorption and emission lines on a continuous background spectrum. The Maxwell-Boltzmann equation gives the number of electrons in any given orbital. The resonance line is then defined as the specific radiation absorbed to reach the excited state. The energies of the various stationary states, or restricted orbits, can then be determined by these emission lines. This is like a fingerprint for an atom, and its called that atoms absorption spectrum. This would manifest itself as dark lines in the spectrum, missing wavelengths or missing energy levels that correspond to the energies of photons that our electron can absorb.
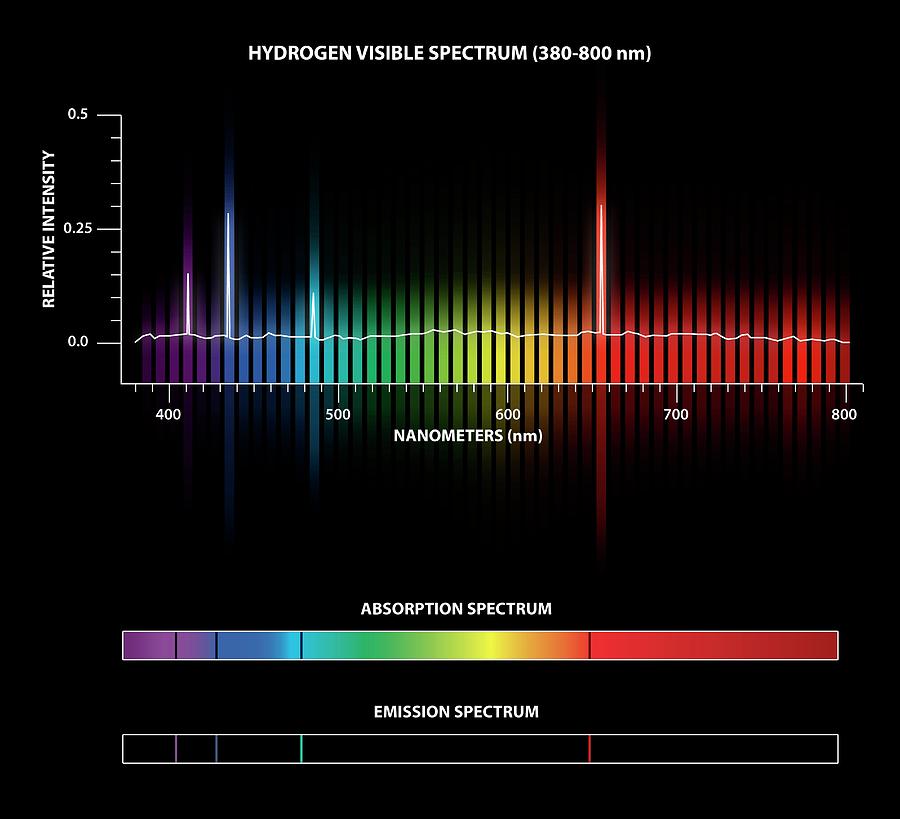
When an atom is excited, the valence electron moves up an energy level. Some of the wavelengths would get absorbed, then scattered away in random directions. Atoms can be excited when irradiated, which creates an absorption spectrum. \): Austrian physicist Wolfgang Pauli (1900 - 1958).Ītoms have valence electrons, which are the outermost electrons of the atom. Refer to the handout " Reference Absorption Spectra." ( Which gases produced this spectrum A.


 0 kommentar(er)
0 kommentar(er)
